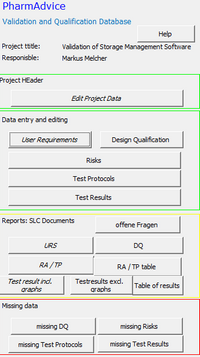
Validation Database
Building on decades of experience in the validation of computerized systems (CSV), PharmAdvice has created a relational database. This effortlessly guarantees the required traceabiliy of user requirements, risk analysis and test plan to test results and significantly accelerates the creation of system life cycle documents.
User Requirements, Functional specification, Risk Analysis, Test Protocol and Testresults are provided as reports at the touch of a button.
Evaluations for missing risks, test plans or test results ensure the completeness of the validation.
For Excel validations, a preconfigured validation database is available with requirements, risks, and test plans for GxP-compliant Excel applications such as
- Audit Trail with justification for changes
- Preventing cutting and pasting in protected register sheets
- Protection against subsequent changes after printing the results
- Record-by-record electronic signatures in GxP tables as protection against unintentional/unjustified changes
- sheet protection, file protection
- Identification of input fields
- Validation checks during data entry
- Conditional formatting to identify unexpected inputs or results (OOS, OOT)
- Documentation of all formulas, VBA code, cell protection, conditional formatting and validation.